Classification of solid fuels
Solid fuels are combustible substances, the main component of which is carbon.
Solid fuels include wood, peat, oil shale, coal and brown coal. The content of carbon, hydrogen, oxygen, nitrogen and sulfur, called chemical composition, determines the properties of solid fuels. When burned, equal amounts of different fuels release different amounts of heat. Then, to assess the calorific value of fuels, the largest amount of heat that can be released by the fuel during its complete combustion in the amount of 1 kg is determined. Coal has the highest calorie content.
Rice. 1. Some types of solid fuel
As is known from the course of thermal engineering, solid organic fuel is often used to produce heat and other types of energy with their subsequent conversion into mechanical energy. In addition, more than 300 different chemical compounds can be obtained from solid fuels with their appropriate distillation (processing).

Rice. 2. Wood is a solid fuel
fuel for stoves
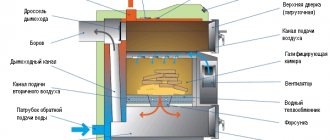
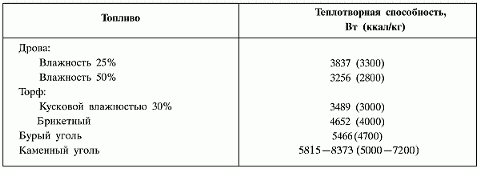
The amount of heat given off by the fuel during combustion and the completeness of combustion of the fuel directly depend on its properties and chemical composition. Typically, any fuel contains hydrogen, carbon, oxygen, mineral additives and free water. Regarding the water content in a particular combustion product, it is worth saying a few words separately. Many are sure that dry fuel is a material that does not contain liquid in principle. We dare to assure you that this confidence is deliberately false: even the driest wood contains 8–10% (by weight) water , not to mention peat or coal. During combustion, carbon combines with hydrogen, the reaction proceeds with the release of a large amount of heat. As in any chemical reaction, the combustion process produces end products, basically two of them. Gaseous - carbon dioxide, which if leaked can pose a serious health hazard (this is where the term “burn” comes from), and water vapor with air nitrogen, which do not take part in combustion. Gaseous combustion products pass through the internal channels of the furnace and escape through the chimney, while they take with them some solid particles, such as soot. Collectively they are called smoke. Solids - mainly coals, soot and ash, passing through the grate, end up in the ash pan, because they no longer take part in the combustion and, remaining in the firebox, can even stop it completely. Some tiny solid particles do not escape and do not end up in the ash pan. They penetrate into the materials from which the stove is made, or settle on the walls of the smoke ducts, clogging them. This is why pipes and smoke ducts need to be cleaned from time to time. The amount of heat released during the combustion process also depends on the degree of combustion of the fuel: the more complete the combustion, the more heat there will be. But no fuel can burn completely in a household stove. However, the degree of combustion can be maximized; to do this, it is necessary to ensure timely removal of combustion reaction products from the furnace and create a good flow of air, since without the oxygen contained in it, combustion will stop. Repairing it yourself will not be easy. The first condition is met due to the presence of a grate, through which, as mentioned above, solid combustion reaction products leave the furnace into the ash pan. As for the air flow, you just need to position the blower door correctly and lay out the chimney of the required height, then the draft effect will provide enough air for complete combustion of the fuel. But proper stove design is not everything. The amount of heat directly depends on the type and quality of fuel used. When burning the same mass, different fuels will release different amounts of heat. The ability of a fuel to release heat during combustion is called calorific value. The calorific values of some fuels are shown in Table 2. Table 2. Calorific values of some fuels
Of course, the most common fuel for home stoves and kitchen hearths is firewood, i.e. wood. The calorific value of firewood depends on the degree of drying and the type of wood from which it was harvested. Thus, oak, birch, beech or maple firewood provides much more heat than, for example, pine, aspen or alder. Properly dried firewood flares up easily, produces almost no smoke (with the exception of resin-containing species such as pine, spruce) and produces little ash. As you probably already understood, such firewood produces fewer combustion products, so it produces more heat. Sometimes it is easier to extract and use peat for heating. Peat is the rotted remains of dry plants that lie in layers in the soil. According to the method of extraction and use, peat is classified into: – carved; – lump; – pressed (in the form of briquettes); – milling (in the form of crumbs). The most valuable and convenient to use is pressed peat . Briquettes have a very high density, so with small volumes of fuel, which is convenient for storage, you can get a large amount of heat. The moisture content of peat ranges from 20 to 40% depending on the degree of drying and the method of processing after its extraction. In terms of calorific value, peat is close to wood, but has a higher ash content. Coal is a compound of carbon, oxygen and hydrogen in a solid state. It is much more expensive and more difficult to extract and process than wood or peat, but has a high calorific value. There are several types of coal and there are corresponding furnaces for each of them, but in any case a grate is required. When burned, the fuel releases part of the thermal energy of the furnace, heating its elements, while part goes along with the smoke into the atmosphere. But for high-quality heating of the room, in addition to using good fuel, you need to ensure that the stove has a high storage capacity. The storage capacity of a stove is the ability of its working surfaces to accumulate and retain heat for a long time, gradually releasing it into the surrounding space (room). If the storage capacity of the furnace is poor, it will have to be constantly heated, which will entail inconvenience in the owner’s time schedule, shorten the life of the furnace and lead to significant excessive consumption of fuel. The next important parameter of the furnace is heat transfer - the amount of heat given off by the furnace to the surrounding space per unit of time. This parameter is important primarily when planning immediately before construction. The heat transfer of the stove depends on its size, wall thickness, materials from which the stove is made, etc. Basically, when planning the design of the stove, it is worth considering that hot gases will move upward, pressing against the upper walls of the smoke ducts. A threshold placed at the bottom will not significantly affect the movement of hot smoke, but a threshold located on the upper wall of the smoke exhaust duct will retain gases and accumulate them to the required limit. In turn, hot gases, lingering in the channels, will give off more heat to the latter.
Coal
Hard coal is coal with a high degree of charring and a gross calorific value of more than 24 MJ/kg (5700 kcal/kg) on an ash-free but wet basis and with a vitrinite reflectance of 0.5 or more.

Rice. 3. Coal
Hard coal includes non-sedimented sludge, not classified. The formation of coals mainly occurred in the Paleozoic, mainly in the Carboniferous period, which is more than 300 million years ago.
Like wood, the chemical composition of coal is a mixture of carbon, hydrogen, oxygen, nitrogen, sulfur, as well as water and volatile substances with small amounts of mineral impurities. Mineral impurities are not subject to combustion and form ash when burning coal. Each of the mined coals differs in the ratio of their constituent components, which also affects their calorific value. A number of organic compounds that make up coal have carcinogenic properties.

Rice. 4. Split piece of coal
For the formation of coal, abundant accumulation of plant matter is necessary. Since the Devonian period, organic matter accumulated in peat bogs, from which fossil coals were formed without oxygen. Most commercial fossil coal deposits date back to the Devonian period, although younger deposits also exist. The oldest coals are estimated to be about 350 million years old.
Coal was one of the first fossil fuels that began to be widely used by humans. The use of coal in industry for energy production has made it possible to take a big step forward. This coal was formed as a sedimentary rock due to the natural decomposition of ancient plants. Coal consists of a huge amount of carbon. The carbon content is several times higher compared to brown coals. In addition, coal contains volatile substances with a low ash content. The most effective alternative type of coal processing is its gasification. During gasification, carbon monoxide and hydrogen are produced from coal, then liquid fuel is produced using catalytic reactions. To obtain one ton of oil-like liquid, it is usually necessary to gasify 2-3 tons of coal. Also, coal is the main raw material for the production of graphite.
Industrially, sulfur, zinc, germanium, lead, and vanadium are extracted from coal. In the production of ceramics, building materials, and abrasives, waste from coal mining and processing, as well as ash remaining after their combustion, is used. In order for coal to be used rationally, mineral impurities are removed from it, that is, coal is enriched. Coke and coal are used in metallurgy in the process of iron smelting. In addition, coal is also used in metallurgy, in the production of steel and cast iron. Hard coal has the highest cost per ton of finished product. This is primarily due to its calorie content and low ash content. Also, coals are often used for heating houses.
We see that coal is used in almost all spheres of life. This circumstance suggests that coal mining will continue for a long time.
The prospects for the development of the coal mining industry around the world are not so clear. There is also no single doctrine for the processing of hard coal chosen by all countries. Strategies for the extraction and processing of coal are developed and adopted by each country individually and depend on the conditions, the level of profitability of the development of coal deposits, geological reserves, environmental restrictions, the place and role of coal in the fuel and energy balance, the degree of integration of the country's economy into regional and global structures and other.

Rice. 5. Coal mining
In a number of countries, due to favorable geological conditions for the exploitation of deposits, the coal industry is a highly profitable industry and serves as an important source of revenue for state budgets. We are talking about countries such as the USA, Australia, South Africa, Canada, Indonesia, Colombia. At the same time, there are many countries where the formerly developed coal mining industry, under the pressure of competition, was recognized as economically viable, as a result of which coal production was stopped, despite the significant increase in the dependence of most of these countries on external supplies of energy resources. This happened in Belgium, Holland, Ireland, Portugal, France, and Japan.
Advantages and disadvantages
Peat briquettes for heating have a number of undeniable advantages, namely:
- safety of use - no sparks are formed, combustion does not emit carcinogenic and toxic fumes;
- the combustible qualities of peat fuel are maintained for several years;
- peat blocks are a natural bioproduct with minimal additives;
- storage requires a significantly smaller area compared to traditional types of fuel (coal, firewood);
- Wholesale purchase of peat briquettes is much cheaper than purchase of diesel fuel, gas, coal;
- The calorific value of peat briquettes occupies an intermediate position between wood and black coal.
But one drawback still exists. It is highly flammable. Therefore, for fire safety reasons, peat fuel is strictly prohibited from being left near fire or heating devices.
Brown coal or lignite
Brown coal or lignite is coal with a low degree of charring that has retained the anatomical structure of the plant matter from which it was formed. This coal has a gross calorific value of less than 24 MJ/kg (5700 kcal/kg) on an ash-free, wet basis. Its vitrinite reflectance is less than 0.5.
Subbitominous coal, or brown coal, is a combustible mineral, fossil coal of the 2nd stage of metamorphism (a transitional link between lignite and hard coal), obtained from lignite or directly from peat.

Rice. 6. A ten-ton lump of lignite at the Lignite Museum in Japan
The classification of fossil coals is quite confusing, for example, in England and the European Union they use the term lignite (which is considered synonymous with brown coal), and in America the concepts of brown coal and lignite are distinguished separately and very clearly. In Russia, a synonym for brown coal is lignite. Basically, this type of coal is called brown coal; lignite with a high degree of carbonization (HCC) also belongs to this category, and without taking into account subbituminous coals with a high degree of carbonization, the latter coals are already classified as hard coal.

Rice. 7. The most typical appearance of brown coal
The use of brown coal in Russia and many other countries for large-scale energy as a fuel is significantly inferior to the use of hard coal. However, its low cost makes it attractive for combustion in small and private boiler houses, where the share of its use averages up to 80%. Brown coal is burned in pulverized (during storage, brown coal dries out and crumbles) and lump forms in a layer. The main energy fuel at thermal power plants in Greece and especially Germany is brown coal to generate electricity. So in Greece, up to 50% of electricity is generated at such stations, 24.6% in Germany.
The production of liquid hydrocarbon fuels from brown coal through distillation is also gaining momentum. After distillation, the residue is suitable for producing soot. Combustible gas is extracted from it, and carbon-alkali reagents and montan wax (mountain wax) are obtained. Montan wax is used in small quantities to make crafts.
If we talk about the stability of the market for thermal coal, it is quite stable. In this connection, an increase in the sales volume of brown is hardly expected. Along with the stable situation in the energy sector, there was a shortage of metallurgical fuels and coke products. And therefore, now many resources are aimed at developing technologies for processing brown coals into a finished product for the needs of metallurgy. Such processing will be economically justified in the future, because the cost of coke products is several times more expensive than ordinary coal.

Rice. 8. Installation that generates electricity from brown coal
In the world, two enterprises have been carbonizing brown coals for many years: the Rheinbraunkole plant with a capacity of 210 thousand tons/year of coke in Germany and a capacity of 80 thousand tons/year. Technologies developed in the 30s of the last century and then improved are characterized by extremely high capital intensity. This aspect makes it impossible to purchase imported technologies and equipment.
In Russia, a fairly large number of scientific teams are working on this topic; there are also technological developments on the topic of thermal upgrading of brown coal. But in most cases, these studies are performed only at the laboratory level
installations. It is known that only 5% of all developments go from laboratory installation to commercial enterprise with reliable technology. This is due to large investments and a long testing period, the return from which will not return very soon.
Calorific value of solid materials
This category includes wood, peat, coke, oil shale, briquette and pulverized fuel. The main component of solid fuel is carbon.
Features of different types of wood
Maximum efficiency from the use of firewood is achieved provided that two conditions are met - dry wood and a slow combustion process.

Pieces of wood are sawn or chopped into pieces up to 25-30 cm long so that firewood can be conveniently loaded into the firebox
Oak, birch, and ash bars are considered ideal for wood-burning stove heating. Hawthorn and hazel are characterized by good indicators. But coniferous trees have a low calorific value, but a high burning rate.
How different rocks burn:
- Beech, birch, ash, and hazel are difficult to melt, but they can burn wet due to their low moisture content.
- Alder and aspen do not form soot and “know how” to remove it from the chimney.
- Birch requires a sufficient amount of air in the firebox, otherwise it will smoke and deposit resin on the walls of the pipe.
- Pine contains more resin than spruce, so it sparks and burns hotter.
- Pear and apple trees split easier than others and burn well.
- The cedar gradually turns into smoldering coal.
- Cherry and elm smoke, and sycamore is difficult to split.
- Linden and poplar burn out quickly.
We recommend: Membrane tank for heating: how to install an expansion tank in a heating system, differences from a hydraulic accumulator, structure and principle of operation, calculation of volume and installation
TST indicators of different breeds strongly depend on the density of specific breeds. 1 cubic meter of firewood is equivalent to approximately 200 liters of liquid fuel and 200 m3 of natural gas. Wood and firewood fall into the low energy efficiency category.
Effect of age on coal properties
Coal is a natural material of plant origin. It is mined from sedimentary rocks. This fuel contains carbon and other chemical elements.
In addition to the type, the heat of combustion of coal is also influenced by the age of the material. Brown belongs to the young category, followed by stone, and anthracite is considered the oldest.

The moisture content is also determined by the age of the fuel: the younger the coal, the higher its moisture content. Which also affects the properties of this type of fuel
The process of coal combustion is accompanied by the release of substances that pollute the environment, and the boiler grates are covered with slag. Another unfavorable factor for the atmosphere is the presence of sulfur in the fuel. This element, upon contact with air, transforms into sulfuric acid.
Manufacturers manage to reduce the sulfur content in coal as much as possible. As a result, TST differs even within the same species. The production geography also influences the performance. Not only pure coal, but also briquetted slag can be used as solid fuel.
The highest fuel capacity is observed in coking coal. Stone, charcoal, brown coal, and anthracite also have good characteristics.
Characteristics of pellets and briquettes
This solid fuel is produced industrially from various wood and plant waste.
Shredded shavings, bark, cardboard, and straw are dried and turned into granules using special equipment. In order for the mass to acquire a certain degree of viscosity, a polymer, lignin, is added to it.

Pellets have an acceptable cost, which is influenced by high demand and features of the manufacturing process. This material can only be used in boilers designed for this type of fuel.
Briquettes differ only in shape; they can be loaded into furnaces and boilers. Both types of fuel are divided into types based on raw materials: round timber, peat, sunflower, straw.
Pellets and briquettes have significant advantages over other types of fuel:
- complete environmental friendliness;
- possibility of storage in almost any conditions;
- resistance to mechanical stress and fungus;
- uniform and long burning;
- optimal granule size for loading into a heating device.
Eco-friendly fuel is a good alternative to traditional heat sources, which are not renewable and have an adverse effect on the environment. But pellets and briquettes are characterized by an increased fire hazard, which should be taken into account when organizing a storage location.
If you wish, you can set up the production of fuel briquettes yourself; more details can be found in this article.
Peat
Peat (German: Torf) is a combustible mineral; formed by the accumulation of moss remains that have undergone incomplete decomposition in swamp conditions. A swamp is characterized by the deposition on the soil surface of incompletely decomposed organic matter, which later turns into peat. The peat layer in swamps is at least 30 cm (if less, then these are wetlands).

Rice. 9. Peat medium-decomposed horizon of sod-podzolic soil-gleyed soil
Peat differs from soil formations in the content of organic compounds; their amount in peat is at least 50% in relation to the absolutely dry mass. In 30-50 years. In the last century, peat was actively used in the energy sector and for gas production, as well as for heating houses.
The use of peat as a fuel is due to its composition: high carbon content, low sulfur content, harmful non-combustible residues and impurities. Essentially, it is young coal.
The main disadvantages of peat are its low calorific value, as well as the difficulty of burning it, which is due to its high moisture content (up to 65%).
The advantages of peat fuel are:
- environmentally friendly combustion (low sulfur content);
- complete combustion (small ash residue);
- low production costs;
- new combustion technologies have emerged.

Rice. 10. Peat in hand
During the marketing research “Russian market of organic fertilizers: results of 2011, forecast for 2012-2013”, conducted by NeoAnalytics, it turned out that the production of peat for agriculture has great prospects. This can be characterized by the fact that the peat industry has declined. Thus, in the period from 1990 to 2011, peat production decreased by more than 20 times, most enterprises associated with the extraction and processing of peat ceased operations, and at other currently operating enterprises, the equipment was physically and morally obsolete. Previously developed peat deposits are overgrown, which increases the cost of peat extraction, or are returned to the state forest fund as unused.

Rice. 11. Properties and applications of peat

Rice. 12. Peat extraction
If we compare peat production indicators, it is easy to notice its decline. Thus, in 2011, 128 thousand tons of peat for agriculture were produced, which is almost 30 times less than production in 1998 (3834 thousand tons). Peat reserves by economic regions of Russia are distributed as follows: more than half of the peat reserves (51%) are located in the West Siberian region, followed by the Northern region in second place (18%), and the Far Eastern region in third place (13%). The smallest peat reserves are located in the Central Economic Region, about 2%.
Peat is considered a renewable natural resource.
It is used mainly in energy and agriculture.
More than 65% of the extracted peat is supplied for agricultural needs. In general, peat resources on a global scale are estimated at more than 400 million hectares. Of these, 162.7 billion tons with a humidity of 40% are located on the territory of the Russian Federation.

Rice. 13. Peat in agriculture
Briquette fuel
Briquette fuel is another purpose of coal briquettes. Briquetting occurs by sintering coal or peat particles, under the influence of temperature and pressure, into briquettes of the correct shape. For better sintering of coal particles, binders are added to coal briquettes during their production.
Peat briquettes are a ready-to-burn product made from raw peat with or without the addition of binders, followed by drying and high-pressure treatment.

Rice. 14. Peat fuel briquettes
Brown coal briquettes are made from brown coal and lignite. They are sintered under high pressure without adding binders after preliminary crushing and drying to form briquettes of the correct shape.
Environmentally friendly fuel.
Nowadays, Euro-firewood (fuel briquettes made from pressed sawdust, shavings, wood dust) is becoming widespread. Euro firewood can be used in stoves of any structure, for heating boilers and fireplaces. Wood briquettes have a number of advantages over conventional firewood. Among the main advantages are high heat transfer, convenient placement, absence of soot and smoke, ease and safety of use.
The most famous and used type of fuel briquettes are products made from pressed dehydrogenated peat. Although they are inferior to European firewood in terms of heat transfer, they have the advantage of a longer burning period, approximately 5-8 hours, depending on the stove heating system used. They also light up quickly and take up minimal space when stored.
There is also a very important feature of Euro-firewood and purified peat briquettes. The products of their combustion can subsequently be used as fertilizer (ash), since they contain virtually no harmful impurities
Coke
Coke is a solid residue obtained by dry distillation of coal or lignite in the complete absence of air access (carbonization).
There are coal coke, lignite coke and gas coke.
6.1 Coal coke
Coal coke (from German Koks and English coke) is a solid, gray, porous product obtained by coking coal at temperatures of 950-1100 °C without air access. Coke contains 96-98% C, the rest H, S, N, O. Porosity 49-53%, true density 1.80-1.95 g/cm³, apparent density ≈ 1 g/cm³, bulk weight 400-500 kg /m³, ash content 9-12%, volatile matter yield 1%. Humidity when extinguishing with water and inert gas is 2-4% and no more than 0.5%, respectively. Ultimate compressive strength 15-25 MPa, shear strength (characterizes abrasion resistance) 6-12 MPa, calorific value 29-30 MJ/kg.

Rice. 15. Coal coke
For the smelting of cast iron, coal coke is mainly used as a high-quality smokeless fuel or blast furnace coke in other words; it is also used as a reducing agent for iron ore and a disintegrant for charge materials. Coal coke is also used as cupola fuel in foundries (foundry coke), for domestic purposes (household coke), and in the chemical and ferroalloy industries (special types of coke).
Blast furnace coke is produced with a piece size of at least 25-40 mm, the presence of fines should be no more than 3% (pieces up to 25 mm and no more than 2-3% for pieces larger than 80 mm.
Foundry coke, if we consider it by the size of the pieces, it turns out that it is larger than blast furnace coke. It is also more suitable as a product containing pieces smaller than 60-80 mm. The main difference between foundry coke and blast coke is that it contains very little sulfur, less than 1% versus 2% in blast furnace coke.
In the production of ferroalloys, coke of fine fractions of the order of 10-25 mm is actively used. The cokes used have a high degree of reactivity. If we talk about the strength of coke, these requirements are less stringent than, for example, for blast furnace or foundry coke.

Rice. 16. Domain process
The best coke for any type of production is strong, low-ash coke with low sulfur content and a small amount of fines.
In the modern world, the production of coal coke is about 550-650 million tons/year. More than half of this volume is produced in China (60-70% of world production).
6.2 Gas coke
Gas coke is a by-product of coal processing, used to produce artificial gas in gas plants, and furnace coke, which includes all other types of coke obtained from coal.
6.3 Lignite coke
Lignite coke is a solid product obtained by carbonization of lignite briquettes.
Rice. 17. Coal particles in lignite coke
Today, the main consumers of lignite coke are ferrous and non-ferrous metallurgy. Here it is used as a reducing agent or process fuel for agglomeration and the production of ferroalloys, as a waste additive in the production of metallurgical coke and the main filler in the production of coke briquettes. The produced coke-coal briquettes are used as household fuel. The main fillers of such coke are heavy or total tar and semi-coke. Semi-cokes are also actively used for their gasification to produce combustible gas and in some chemical industries.
Calorific value of various types of fuel. Comparative analysis
(Fig. 14.1 – Fuel calorific value)
Pay attention to the calorific value (specific heat of combustion) of various types of fuel, compare the indicators. The calorific value of fuel characterizes the amount of heat released during complete combustion of fuel weighing 1 kg or volume 1 m³ (1 l). Most often, calorific value is measured in J/kg (J/m³; J/l). The higher the specific heat of combustion of the fuel, the lower its consumption. Therefore, calorific value is one of the most significant characteristics of fuel. The specific heat of combustion of each type of fuel depends on:
- From its flammable components (carbon, hydrogen, volatile combustible sulfur, etc.).
- From its moisture and ash content.
| Table 4 - Specific heat of combustion of various energy carriers, comparative analysis of costs. | |||||||||
| Type of energy carrier | Calorific value | Volume density of the substance (ρ=m/V) | Price per unit of standard fuel | Coeff. efficiency (efficiency) of the heating system, % | Price per 1 kWh | Implemented systems | |||
| MJ | kWh | ||||||||
| (1MJ=0.278kWh) | |||||||||
| Electricity | — | 1.0 kWh | — | 3.70 rub. per kWh | 98% | 3.78 rub. | Heating, hot water supply (DHW), air conditioning, cooking | ||
| Methane (CH4, boiling point: -161.6 °C) | 39.8 MJ/m³ | 11.1 kWh/m³ | 0.72 kg/m³ | 5.20 rub. per m³ | 94% | 0.50 rub. | Heating, hot water supply (DHW), cooking, backup and permanent power supply, autonomous septic tank (sewerage), outdoor infrared heaters, outdoor barbecues, fireplaces, baths, designer lighting | ||
| Propane (C3H8, boiling point: -42.1 °C) | 46.34 MJ/kg | 23.63 MJ/l | 12.88 kWh/kg | 6.57 kWh/l | 0.51 kg/l | 18.00 rub. hall | 94% | 2.91 rub. | Heating, hot water supply (DHW), cooking, backup and permanent power supply, autonomous septic tank (sewerage), outdoor infrared heaters, outdoor barbecues, fireplaces, baths, designer lighting |
| Butane C4H10, boiling point: -0.5 °C) | 47.20 MJ/kg | 27.38 MJ/l | 13.12 kWh/kg | 7.61 kWh/l | 0.58 kg/l | 14.00 rub. hall | 94% | 1.96 rub. | Heating, hot water supply (DHW), cooking, backup and permanent power supply, autonomous septic tank (sewerage), outdoor infrared heaters, outdoor barbecues, fireplaces, baths, designer lighting |
| Propane-butane (LPG - liquefied petroleum gas) | 46.8 MJ/kg | 25.3 MJ/l | 13.0 kWh/kg | 7.0 kWh/l | 0.54 kg/l | 16.00 rub. hall | 94% | 2.42 rub. | Heating, hot water supply (DHW), cooking, backup and permanent power supply, autonomous septic tank (sewerage), outdoor infrared heaters, outdoor barbecues, fireplaces, baths, designer lighting |
| Diesel fuel | 42.7 MJ/kg | 11.9 kWh/kg | 0.85 kg/l | 30.00 rub. per kg | 92% | 2.75 rub. | Heating (heating water and generating electricity is very expensive) | ||
| Firewood (birch, humidity - 12%) | 15.0 MJ/kg | 4.2 kWh/kg | 0.47-0.72 kg/dm³ | 3.00 rub. per kg | 90% | 0.80 rub. | Heating (inconvenient to cook food, almost impossible to get hot water) | ||
| Coal | 22.0 MJ/kg | 6.1 kWh/kg | 1200-1500 kg/m³ | 7.70 rub. per kg | 90% | 1.40 rub. | Heating | ||
| MAPP gas (a mixture of liquefied petroleum gas - 56% with methyl acetylene-propadiene - 44%) | 89.6 MJ/kg | 24.9 kWh/m³ | 0.1137 kg/dm³ | -R. per m³ | 0% | Heating, hot water supply (DHW), cooking, backup and permanent power supply, autonomous septic tank (sewerage), outdoor infrared heaters, outdoor barbecues, fireplaces, baths, designer lighting | |||
(Fig. 14.2 - Specific heat of combustion)
According to the table “Specific Heat of Combustion of Various Energy Carriers, Comparative Analysis of Costs,” propane-butane (liquefied petroleum gas) is inferior in economic benefits and prospects for use only to natural gas (methane). However, attention should be paid to the tendency towards an inevitable increase in the cost of main gas, which is currently significantly underestimated. Analysts predict an inevitable reorganization of the industry, which will lead to a significant increase in the price of natural gas, perhaps even exceeding the cost of diesel fuel. Thus, liquefied petroleum gas, the cost of which will remain virtually unchanged, remains extremely promising - the optimal solution for autonomous gasification systems.
Oil shale
Oil shale is a sedimentary rock high in organic matter (kerogen) that can be converted into crude oil or gas by heating.
Oil shale is a mineral from the group of solid caustobiolites, which during dry distillation produces a significant amount of resin (close in composition to oil). The formation of shales mainly occurred 450 million years ago on the seabed from plant and animal remains.
Oil shale consists of predominant mineral (kaolinite, calcite, montmorillonite, quartz, feldspars, dolomite, hydromica, pyrite, etc.) and organic parts (kerogen), the latter making up 10-30% of the rock mass and only in shale of the highest quality reaches 50-70%. The organic part is a bio- and geochemically transformed substance of protozoan algae that has retained its cellular structure (thallomoalginite) or lost it (colloalginite); As an impurity, the organic part contains altered remains of higher plants (vitrinite, fusainite, lipoidinite).

Rice. 18. Kukersite (oil shale).
Oil shale is a rock of mixed clastic and organic origin; are formed at the bottom of seas, lagoons, lakes with the simultaneous deposition of clay particles, carbonate matter and sapropelic silt with organic residues (plankton, higher plants) under conditions of limited circulation of water and air. The accumulated organic-mineral mass gradually compacts and transforms into dense sedimentary rock.
Oil shale has established itself primarily as a very valuable energy raw material. They are used as fuel, as well as in various industries: the chemical industry, agriculture and road construction, in the energy sector, and in the production of building materials. Shale resin is of particular value. It is used as a raw material for the production of liquid fuel, as well as various valuable chemical products (drying oil, sulfur, pesticides, paints). Synthetic oil is also sometimes produced from oil shale. They also contain significant concentrations of radioactive and rare earth elements.
These rocks are divided, in particular, into combustible, clayey and crystalline. The daily range of uses for shale is very large. For example, slate rocks are used for the production of fire-resistant raw materials, in construction for exterior decoration, and the well-known tiles also belong to the same rock.
Shale gas is extracted from rocks located at great depths. Basically, such gas is stored in shale, which has a porous structure. The gas content in shale is small and it is stored there in small portions in the industrial sense. Thus, when gas is pumped out of shale rocks, many impurities are introduced into it. Today, the methane content in produced gas varies from 30 to 70%. This circumstance indicates the need to purify the gas in the already complex process of gas production. Therefore, in order to produce inexpensive, but at the same time clean gas, they are trying to develop gas deposits shaped like bubbles.
According to the international energy agency IEA and the independent energy consulting company ARI, as of June 2013, the largest reserves of shale gas are located in the United States - about 32.875 billion cubic meters. China is in second place - according to experts, 31.573 billion cubic meters are concentrated there. In Europe, significant reserves of shale gas have been discovered in Austria, Great Britain, Hungary, Germany, Sweden, Ukraine and Poland. Russia ranks only 9th in this ranking, but is by far the leader in the list of shale oil owners.
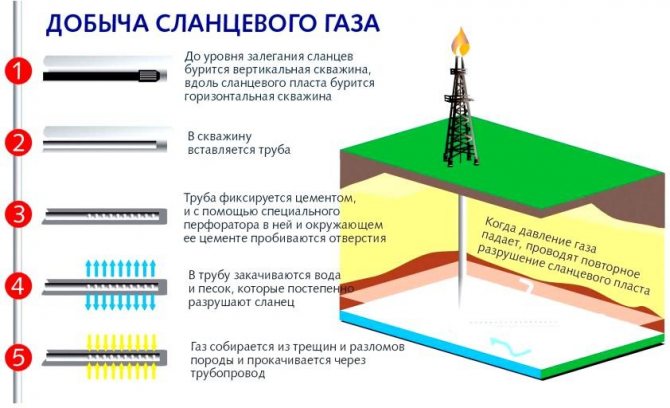
Rice. 19. Shale gas production scheme
Today, shale gas production has been banned in a number of European countries by introducing a moratorium. This is due to concern for the environment. Russia also stated that it does not intend to begin developing shale gas in the coming decades. The United States, on the contrary, has been producing shale gas for several years now. World shale gas reserves are currently estimated at 220.729 billion cubic meters.
Tar sands
Tar sands are sands or sandstones (bitumens) with a high content of resinous carbons that are capable of releasing oil when heated or other extraction processes.
Tar sands also include other types of crude oil, as well as thick, viscous petroleum products with high density and viscosity. These bitumens, or more correctly tar sands, cannot be produced by the traditional method of oil production, that is, by natural flowing or pumping. The reason for this lies precisely in their density and viscosity. To extract them, they strive to reduce their viscosity by heating the hard rocks containing them, and thereby separate them. In addition, many other special methods are used to extract oil from tar sands.
Heavy crude oils are also classified as solid fuels. The parameter separating crude oil and bitumen is their viscosity. When talking about distinguishing between extra-heavy crude oil, heavy crude oil and other types of oil, it is necessary to evaluate their density.

Rice. 20. Photo of tar sands
The oil sands of Venezuela and Canada contain large oil reserves of about 3,400 billion barrels. The development of these deposits is carried out mainly by quarry or mine methods. Today, leading companies such as Shell and BP are still unable to offer technology that would allow large volumes of oil to be extracted from the oil sands. However, this does not stop them in their search and they still continue their research.
Oil reserves in the tar sands of Alberta (Canada) and Orinoco (Venezuela) are 1.7 and 2.0 trillion, respectively. barrels, while world reserves of conventional oil at the beginning of 2006 were estimated at 1.1 trillion. barrels. Oil production in 2006 from Alberta's tar sands was 1.126 Mb/d (million barrels per day). Oil production from the Orinoco tar sands is 0.5 Mb/d. All world oil production is about 84 Mb/d. Thus, although tar sands reserves are huge, oil production from them in the foreseeable future (according to current forecasts) will satisfy only a few percent of the world's oil needs. The problem is that current technologies for extracting oil from tar sands require large amounts of fresh water.
Technology
The most suitable raw material for the production of peat briquettes is low-grade peat, which does not contain any by-products and consists of fine particles.
Fuel bricks from such low-lying peat bog are high-calorie and dense, dark brown, almost black in color.
The technology for producing peat fuel is quite simple and is as follows:
- peat chips are extracted by milling the upper layer of caustobiolite;
- using drying and turning, the crushed raw materials are brought to the required moisture content;
- Then the dried products are stored in piles.
The next production stage is drying the semi-finished product to 9-12 percent humidity. Then the raw materials are separated and sent to the press for briquetting. High pressure and temperature conditions of 200-350 degrees Celsius contribute to the melting of workpieces, increasing their strength.
The result is a peat briquette with certain parameters:
- briquette size – 150 x 70 x 60 mm;
- mass fraction of sulfur – within 0.2%;
- content of ash impurities – no more than 15%;
- standard humidity – within 18%;
- calorific value – from 4500 Kcal/kg.
This briquetted fuel is popular among private owners and is used in furnaces and boilers with controlled air supply. And the ash obtained from the combustion of peat briquettes, according to reviews from amateur gardeners, is an ideal mineral fertilizer with a high content of potassium and phosphorus.
You can read about the boiler room in a private house in this article.
In addition to the milling method of extracting peat raw materials, there is also a less expensive and simple method for harvesting sod peat. With the help of special attachments to the tractor, compaction takes place in areas where high-moor peat is being developed.